.jpg)
.jpg)
(Head Pictures)

Proteins & Surfaces
Unravel Protein Adsorption at Solid Surfaces
Protein adsorption at solid surfaces plays a key role in many natural processes and has therefore promoted a widespread interest in many research areas. Despite consid
erable progress in this field there are still widely differing and even
contradictive opinions on how to explain the phenomena that are frequently
observed. The goal of the project is to understand protein adsorption and to
systematically unravel the underlying molecular mechanism. This is achieved by
acquiring and evaluating comprehensive experimental data sets using fluorescence
sensing and imaging methods. Experiments are conducted on model systems
comprising the proteins BSA, Fibrinogen, β-Lactoglobulin, and a-Synuclein,
hydrophilic and hydrophobic surfaces and varying pH and ionic strength
conditions.
One of the comprehensively studied adsorption phenomena is cooperativity which
refers to the effect that the adsorption of proteins is enhanced by the presence
of pre-adsorbed proteins. Contradicting a widespread opinion we could show for
the first time that cooperativity is not necessarily associated with the growth
of tight surface aggregates. Instead, a macroscopic model description is
suggested that simply assumes the overlap of two parallel adsorption pathways,
one for the adsorption at isolated surface positions and one for the adsorption
near other surface-bound proteins.
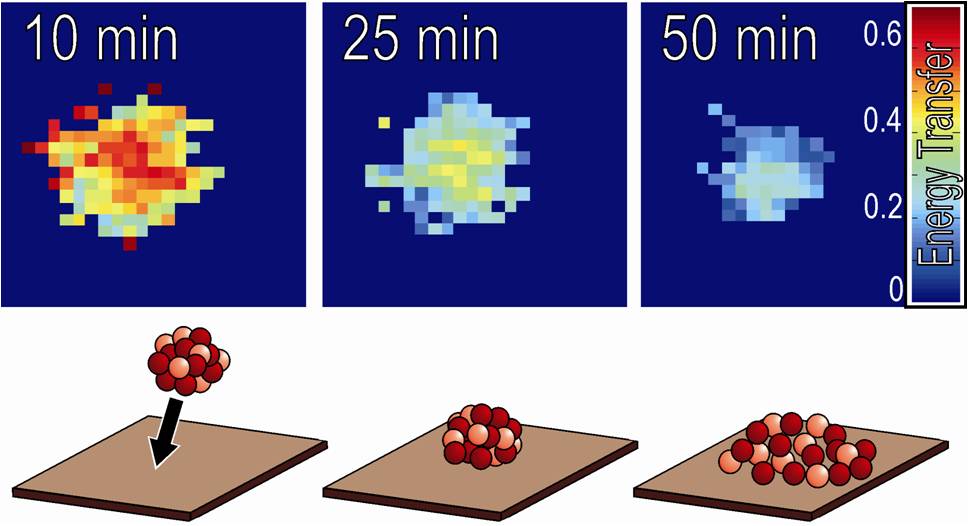
Finally, the behavior of protein aggregates or clusters on surfaces is explored.
Protein clusters can form spontaneously in the solution and subsequently deposit
onto the surface. For the first time it was shown that induced by
protein-surface interactions freshly deposited protein clusters start to spread
in order to maximize the contact area between proteins and surface. The
spreading rate is considerably faster on hydrophobic surfaces as compared to
hydrophilic surfaces which correlate with the lateral mobility of the protein
monomers on theses surfaces. Interestingly, on a hydrophobic surface a spreading
protein cluster can even rupture a pre-adsorbed protein monolayer by displacing
the monomers from the area that it is about to occupy. Inversely to protein
aggregation in solution, the direct growth of aggregates on the surface can also
be observed using the protein a-Synuclein
which is the pathological component of Parkinson’s disease. Whereas the
on-surface growth mechanism is the typically proposed one when protein
aggregates are detected on a surface, the discovery that protein aggregates can
also come from the solution and spread on the surface opens a completely new
perspective on this topic. Experimental strategies to distinguish between these
two different mechanisms are comprehensively discussed.